Bromide Ion Reducing . Halide ions can also act as reducing agents and donate electrons to another atom. Reducing agents are electron donors. Iodide ions reduce the sulphuric acid to a mixture of products including. Oxidation numbers versus the true charge on ions. In each case, a halogen higher in the group can oxidise the ions of one lower down. Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Reducing ability of the halide ions. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously. Reduction occurs when the oxidation number of an atom becomes smaller. The halide ions themselves get oxidised and lose electrons. Halide ions as reducing agents. Bromide ions reduce the sulphuric acid to sulphur dioxide. Bromide is a strong enough reducing agent to reduce sulfuric acid. In the process, the bromide ions are oxidised to bromine.
from www.shutterstock.com
Reducing ability of the halide ions. Reducing agents are electron donors. Bromide is a strong enough reducing agent to reduce sulfuric acid. In each case, a halogen higher in the group can oxidise the ions of one lower down. Bromide ions reduce the sulphuric acid to sulphur dioxide. Oxidation numbers versus the true charge on ions. Halide ions as reducing agents. In the process, the bromide ions are oxidised to bromine. Iodide ions reduce the sulphuric acid to a mixture of products including. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously.
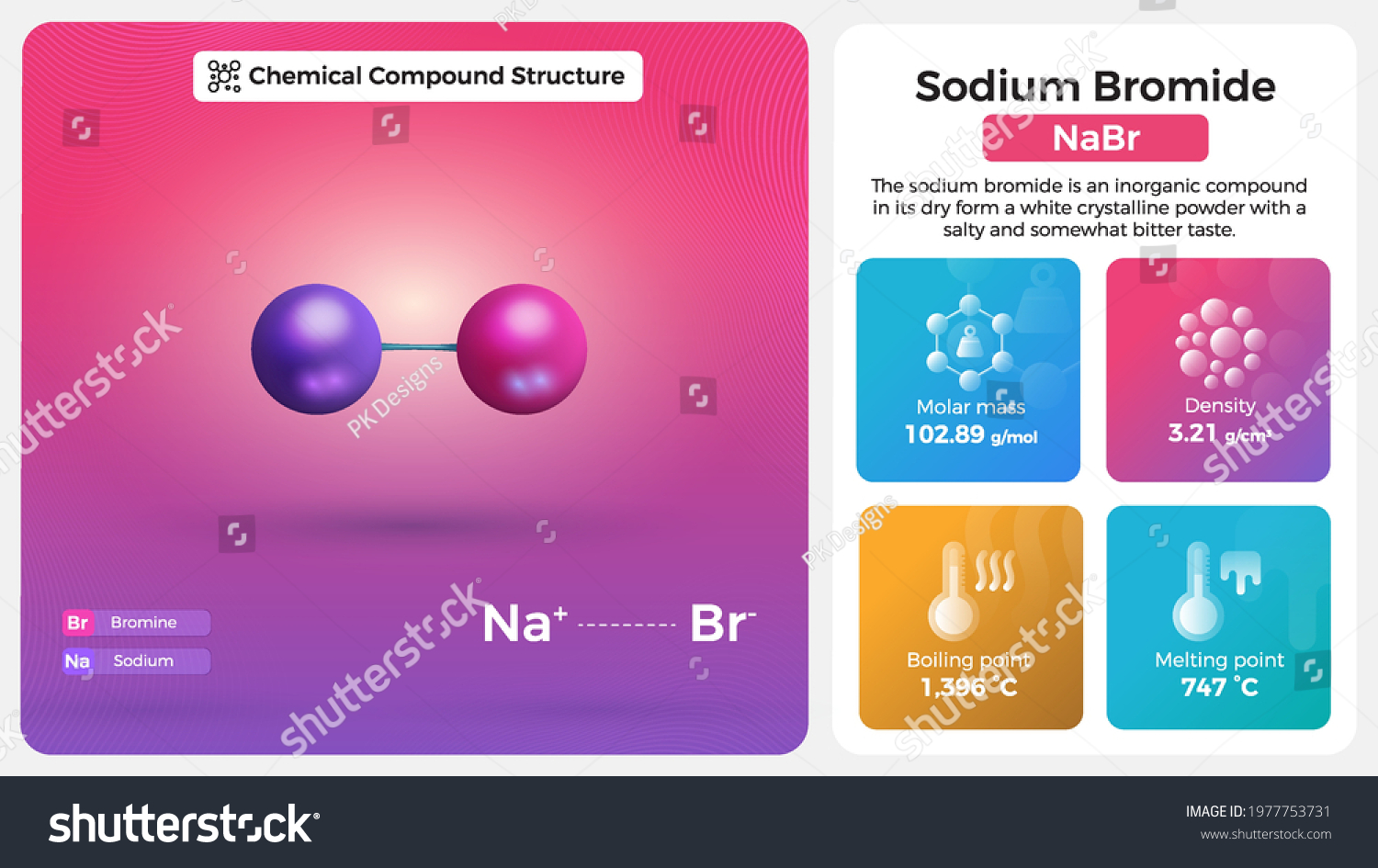
Sodium Bromide Properties Chemical Compound Structure Stock Vector
Bromide Ion Reducing Reducing ability of the halide ions. Bromide is a strong enough reducing agent to reduce sulfuric acid. In each case, a halogen higher in the group can oxidise the ions of one lower down. Halide ions as reducing agents. Iodide ions reduce the sulphuric acid to a mixture of products including. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously. Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. The halide ions themselves get oxidised and lose electrons. Reducing agents are electron donors. Bromide ions reduce the sulphuric acid to sulphur dioxide. Reducing ability of the halide ions. Reduction occurs when the oxidation number of an atom becomes smaller. In the process, the bromide ions are oxidised to bromine. Oxidation numbers versus the true charge on ions. Halide ions can also act as reducing agents and donate electrons to another atom.
From www.numerade.com
SOLVED How does an atom of bromine79 a bromide ion with a 1 Bromide Ion Reducing Oxidation numbers versus the true charge on ions. Bromide is a strong enough reducing agent to reduce sulfuric acid. Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Halide ions can also act as reducing agents and donate electrons to another atom. In each case,. Bromide Ion Reducing.
From www.coursehero.com
[Solved] . 7. A student was trying to figure out whether isopropyl Bromide Ion Reducing In the process, the bromide ions are oxidised to bromine. Reducing agents are electron donors. The halide ions themselves get oxidised and lose electrons. Bromide is a strong enough reducing agent to reduce sulfuric acid. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur. Bromide Ion Reducing.
From brainly.com
PLEASE HELP Which particle represents the size of the bromide ion Bromide Ion Reducing Reducing agents are electron donors. In each case, a halogen higher in the group can oxidise the ions of one lower down. Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Bromide ions reduce the sulphuric acid to sulphur dioxide. Iodide ions reduce the sulphuric. Bromide Ion Reducing.
From www.nagwa.com
Question Video Identifying the Major Product of the Reaction of Bromide Ion Reducing Halide ions can also act as reducing agents and donate electrons to another atom. In each case, a halogen higher in the group can oxidise the ions of one lower down. The halide ions themselves get oxidised and lose electrons. In the process, the bromide ions are oxidised to bromine. Oxidation numbers versus the true charge on ions. Halide ions. Bromide Ion Reducing.
From www.researchgate.net
Correlation between concentration of bromide ion solution and amount of Bromide Ion Reducing In each case, a halogen higher in the group can oxidise the ions of one lower down. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously. Bromide ions reduce the sulphuric acid to sulphur dioxide. Halide ions as reducing agents. Bromide is a. Bromide Ion Reducing.
From cartoondealer.com
Aclidinium Bromide Molecule, Structural Chemical Formula, Balland Bromide Ion Reducing Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Iodide ions reduce the sulphuric acid to a mixture of products including. Halide ions as reducing agents. In the process, the bromide ions are oxidised to bromine. In each case, a halogen higher in the group. Bromide Ion Reducing.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Bromide Ion Reducing Halide ions as reducing agents. The halide ions themselves get oxidised and lose electrons. Bromide ions reduce the sulphuric acid to sulphur dioxide. In each case, a halogen higher in the group can oxidise the ions of one lower down. Iodide ions reduce the sulphuric acid to a mixture of products including. Reducing agents are electron donors. Reducing ability of. Bromide Ion Reducing.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Bromide Ion Reducing Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously. Bromide ions reduce the sulphuric acid to sulphur dioxide. Bromide is a strong enough reducing agent to reduce sulfuric acid. Reduction occurs when the oxidation number of an atom becomes smaller. Reducing ability of. Bromide Ion Reducing.
From cartoondealer.com
Aclidinium Bromide Molecule, Structural Chemical Formula, Balland Bromide Ion Reducing The halide ions themselves get oxidised and lose electrons. Bromide is a strong enough reducing agent to reduce sulfuric acid. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value of e° cell, will occur spontaneously. Reducing agents are electron donors. Bromide ions reduce the sulphuric acid to sulphur dioxide.. Bromide Ion Reducing.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromide Ion Reducing Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Bromide ions reduce the sulphuric acid to sulphur dioxide. Halide ions as reducing agents. In the process, the bromide ions are oxidised to bromine. Bromide is a strong enough reducing agent to reduce sulfuric acid. Iodide. Bromide Ion Reducing.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Bromide Ion Reducing In each case, a halogen higher in the group can oxidise the ions of one lower down. Oxidation numbers versus the true charge on ions. Halide ions can also act as reducing agents and donate electrons to another atom. The halide ions themselves get oxidised and lose electrons. Halide ions as reducing agents. Reducing agents are electron donors. Instead, the. Bromide Ion Reducing.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记2.3.4 Halide Ion Reactions翰林国际教育 Bromide Ion Reducing Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Iodide ions reduce the sulphuric acid to a mixture of products including. Oxidation numbers versus the true charge on ions. Bromide is a strong enough reducing agent to reduce sulfuric acid. Reducing ability of the halide. Bromide Ion Reducing.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide Ion Reducing Bromide ions reduce the sulphuric acid to sulphur dioxide. In the process, the bromide ions are oxidised to bromine. In each case, a halogen higher in the group can oxidise the ions of one lower down. Halide ions can also act as reducing agents and donate electrons to another atom. Bromide ions are stronger reducing agents than chloride ions, they. Bromide Ion Reducing.
From www.sarthaks.com
How will you synthesise ? (a) Isopropyl bromide from npropyl bromide Bromide Ion Reducing In each case, a halogen higher in the group can oxidise the ions of one lower down. Iodide ions reduce the sulphuric acid to a mixture of products including. Reducing agents are electron donors. In the process, the bromide ions are oxidised to bromine. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which. Bromide Ion Reducing.
From www.mcguffmedical.com
Ipratropium Bromide, 0.02, Inhalation Solution, 2.5mL, 25 Vials/Tray Bromide Ion Reducing Halide ions can also act as reducing agents and donate electrons to another atom. In each case, a halogen higher in the group can oxidise the ions of one lower down. In the process, the bromide ions are oxidised to bromine. Bromide is a strong enough reducing agent to reduce sulfuric acid. The halide ions themselves get oxidised and lose. Bromide Ion Reducing.
From hamptonresearch.com
Hampton Research Bromide Ion Reducing Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Bromide is a strong enough reducing agent to reduce sulfuric acid. Reducing ability of the halide ions. Reduction occurs when the oxidation number of an atom becomes smaller. In the process, the bromide ions are oxidised. Bromide Ion Reducing.
From sinoleadbio.en.made-in-china.com
Rocuronium Bromide Injection 5ml 50mg. 5ampoules/Box China Bromide Ion Reducing In each case, a halogen higher in the group can oxidise the ions of one lower down. Oxidation numbers versus the true charge on ions. Bromide ions are stronger reducing agents than chloride ions, they can reduce the sulfur from a +6 oxidation state to a +4 oxidation state. Bromide is a strong enough reducing agent to reduce sulfuric acid.. Bromide Ion Reducing.
From www.alamy.com
Bromine reaction. Bromine gas (orange) forming as the product of a Bromide Ion Reducing Oxidation numbers versus the true charge on ions. Reduction occurs when the oxidation number of an atom becomes smaller. The halide ions themselves get oxidised and lose electrons. Iodide ions reduce the sulphuric acid to a mixture of products including. Instead, the reverse process, the reduction of stannous ions (sn 2 +) by metallic beryllium, which has a positive value. Bromide Ion Reducing.